
- Chun-Woong Park
Education & Career
- 2005 - 2008 : Ph.D. in Pharmaceutics, Sungkyunkwan University, Suwon, South Korea.
- 2003 - 2005 : M.S. in Pharmaceutics, Sungkyunkwan University, Suwon, South Korea.
- 1997 - 2003 : B.S. in Pharmacy, Sungkyunkwan University, Suwon, South Korea.
- Sept 2012 – Present : Assistant Professor, College of Pharmacy, Chungbuk National University
- Nov 2011 – Aug 2012 : Research Associate, School of Pharmacy, Sungkyunkwan University
- May 2010 – Aug 2011: Visiting Postdoctoral Fellow, University of Kentucky College of Pharmacy
- Sept 2008 – Apr 2010 : Research Associate, School of Pharmacy, Sungkyunkwan University
Research Areas
-
Lung-targeted Dry Powder Inhalation (DPI)
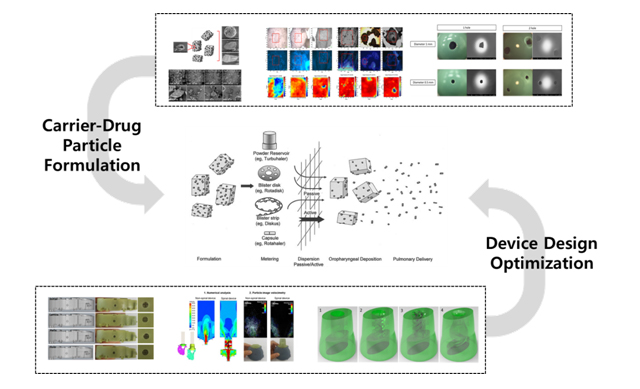
-
Transdermal/Dermal-targeted formulation development
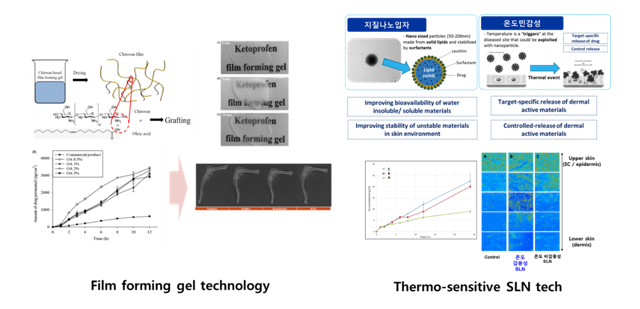
-
Microparticle engineering for pharmaceutical dosage forms

Selected Publications
- Hyo-Jung Lee, In-Ho Kwon, Hong-Goo Lee, Yong-Bin Kwon, Hye-Min Woo, Sang-Min Cho, Youn-Woong Choi, Jinmann Chon, Kibum Kim, Dong-Wook Kim*, Chun-Woong Park*, Spiral mouthpiece design in a dry powder inhaler to improve aerosolization, International journal of pharmaceutics (IN PRESS) *Co-corresponding authors to this work
- Thi-Tram Nguyen, Eun-Jin Yi, Kyu-Mok Hwang, Cheol-Hee Cho, Chun-Woong Park, Ju-Young Kim, Yun-Seok Rhee, Eun-Seok Park, Formulation and evaluation of carrier-free dry powder inhaler containing sildenafil, Drug delivery and translational research (IN PRESS)
- Ji-Hun An, Wonno Youn, Alice Kiyonga, Changjin Lim, Minho Park, Young-Ger Suh, Hyung Ryu, Jae Kim, Chun-Woong Park*, Kiwon Jung*, Kinetics of the Solution-Mediated Polymorphic Transformation of the Novel l-Carnitine Orotate Polymorph, Form-II, Pharmaceutics 10 (4), 171 (2018) *Co-corresponding authors to this work
- Hyo-Jung Lee, Hong-Goo Lee, Yong-Bin Kwon, Ju-Young Kim, Yun-Seok Rhee, Jinmann Chon, Eun-Soek Park, Dong-Wook Kim*, Chun-Woong Park*, The role of lactose carrier on the powder behavior and aerodynamic performance of bosentan microparticles for dry powder inhalation, European Journal of Pharmaceutical Sciences 117, 279-289 (2018) *Co-corresponding authors to this work
- Chun-Woong Park, Hyo-Jung Lee, Dong-Won Oh, Ji-Hyun Kang, Chang-Soo Han, Dong-Wook Kim, Preparation and in vitro/in vivo evaluation of PLGA microspheres containing norquetiapine for long-acting injection, Drug Design, Development and Therapy 12, 711-719 (2018)
- Jung-Myung Ha, Jeong-Woong Seo, Su-Hyeon Kim, Ju-Young Kim, Chun-Woong Park, Yun-Seok Rhee, Eun-Seok Park, Implementation of quality by design for formulation of rebamipide gastro-retentive tablet, AAPS PharmSciTech 18 (8), 3129-3139 (2017)
- Su-Hyeon Kim, Kyu-Min Hwang, Cheol-Hee Cho, Thi-Tram Nguyen, Su Hyun Seok, Kyu-Mok Hwang, Ju-Young Kim, Chun-Woong Park, Yun-Seok Rhee, Eun-Seok Park, Application of continuous twin screw granulation for the metformin hydrochloride extended release formulation, International journal of pharmaceutics 529 (1-2), 410-422 (2017)
-
Hyo-Jung Lee, Dong-Won Oh, Min-Ju Na, Dong-Wook Kim, Dong-Yeon Yuk, Hyoung-Chul Choi, Yong-Beom Lee, Kun Han*, Chun-Woong Park *, Preparation and in vivo evaluation of lecithin-ba
sed microparticles for topical delivery of minoxidil, Archives of pharmacal research 40 (8), 943-951 (2017) *Co-corresponding authors to this work -
Dae Hwan Shin, So Young Lee, Sung Woo Jeong, Chun-Woong Park, Youn Bok Chung, Interspecies pharmacokinetic scaling of 11-hydroxyaclanomycin X ba
sed on animal data, Journal of Biomedical and Translational Research 18 (1), 24-29 (2017) - Hong-Goo Lee, Dong-Wook Kim, Chun-Woong Park, Dry powder inhaler for pulmonary drug delivery: human respiratory system, approved products and therapeutic equivalence guideline, Journal of Pharmaceutical Investigation (IN PRESS)
-
Dong-Won Oh, Ji-Hyun Kang, Hyo-Jung Lee, Sang-Duk Han, Min-Hyung Kang, Yie-Hyuk Kwon, Joon-Ho Jun, Dong-Wook Kim, Yun-Seok Rhee, Ju-Young Kim, Eun-Seok Park, Chun-Woong Park, Formulation and in vitro/in vivo evaluation of chitosan-ba
sed film forming gel containing ketoprofen, Drug delivery 24 (1), 1056-1066 (2017) - Hun Jun, Hyo-Jung Lee, Beom-Soo Shin, Chun-Woong Park, Preparation and in vivo characterization of dual release tablet containing sarpogrelate hydrochloride, Journal of Pharmaceutical Investigation (IN PRESS)
- Hyo-Jung Lee, Ju-Young Kim, Sung-Hoon Park, Yun-Seok Rhee, Chun-Woong Park*, Eun-Seok Park*, Controlled-release oral dosage forms containing nimodipine solid dispersion and hydrophilic carriers, Journal of Drug Delivery Science and Technology 37(2), 28-37 (2017) *Co-corresponding authors to this work
- Hyo-Jung Lee, Ji-Hyun Kang, Hong-Goo Lee, Dong-Wook Kim, Yun-Seok Rhee, Ju-Young Kim, Eun-Seok Park*, Chun-Woong Park*, Preparation and physicochemical characterization of spray-dried and jet-milled microparticles containing bosentan hydrate for dry powder inhalation aerosols, Drug Design Development and Therapy 10, 4017-4030 (2016) *Co-corresponding authors to this work
- Eon-Pyo Hong, Ju-Young Kim, Su-Hyeon Kim, Kyu-Mok Hwang, Chun-Woong Park, Hyo-Jung Lee, Dong-Wook Kim, Kwon-Yeon Weon, Seo Young Jeong, Eun-Seok Park, Formulation and evaluation of a self-microemulsifying drug delivery system containing bortezomib, Chemical and Pharmaceutical Bulletin 64(8), 1108-1117 (2016)
-
Su-Hyeon Kim, Tack-Oon Oh, Ju-Young Kim, Chun-Woong Park, Seung-Ho Baek, Eun-Seok Park, Effects of me
tal-and fiber-reinforced composite root canal posts on flexural properties, Dental materials journal 35(1), 138-146 (2016) - Kyu-Mok Hwang, Shin-Ae Park, Ju-Young Kim, Chun-Woong Park, Yun-Seok Rhee, Eun-Seok Park, Formulation and in vitro evaluation of self-microemulsifying drug delivery system containing fixed-dose combination of atorvastatin and ezetimibe, Chemical and Pharmaceutical Bulletin 63(6), 423-430 (2015)
- Ju-Young Kim, Chun-Woong Park, Beom-Jin Lee, Eun-Seok Park and Yun-Seok Rhee, Design and evaluation of nicorandil extended-release tablet, Asian J. Pharm Sci., 10 (2), 108-113 (2015)
- Ju-Young Kim, Sung-Hoon Lee, Chun-Woong Park, Yun-Seok Rhee, Dong-Wook Kim, Junsang Park, Moonseok Lee, Jeong-Woong Seo and Eun-Seok Park, Design and in vivo evaluation of oxycodone once-a-day controlled-release tablets, Drug Des. Devel. Ther., 9, 707-716 (2015)
- Ju-Young Kim, Kyu-Mok Hwang, Chun-Woong Park, Yun-Seok Rhee, Eun-Seok Park, Organic-aqueous crossover coating process for the desmopressin orally disintegrating microparticles, Drug Dev. Ind. Pharm., 41(2), 292-299 (2015)
Patents
- 1. Stability improved composite particles containing fesoterodine fumarate and oral dosage form comprising the same, Korean Patent Number 10-1808963-0000 (2017)
-
Taste-masking composite particles containing donepezil freeba
se and oral dosage form comprising the same, Korean Patent Number 10-1791342-0000 (2017) - A pharmaceutical composition comprising minoxidil, Korean Patent Number 10-1766752-0000 (2017)
Industrial collaboration
- Formulation of MNX liposomal product (2015; TOTAL HEALTH POINT)
- Formulation of PMX fixed dose combination (2015; CRYSTALGENOMICS)
- Formulation of DWJ133 extended-release oral dosage form (2013-2015; DAEWOONG PHARM)
- Formulation of IT102 fixed-dose combination product (2015-2016; ITHENAPHARM)
- Formulation of DNP orally disintegrating product (2015-2016; COREPHARM BIO)
- Formulation of CLD / BZP topical gel (2015; HPNC)
- Feasibility study of KTP film-forming gel (2016; DONG-A PHARM)
- Formulation of TCR topical gel (2016; HPNC)
- Feasibility study of iontophoresis system (2017; DONG-A PHARM)
- PK-PD study of AFM/BUD fixed-dose DPI product (2017-2018; KOREA UNITED PHARM)
- Optimization study of DPI device design (2017; KOREA UNITED PHARM)
- Formulation of BST inhalable microparticles for DPI (2017-2018; MOTHERS PHARM)
- Formulation of PRX transdermal patch (2017; JEIL HEALTH SCIENCE)
- PD study of scar gel product (2018; DONG-A PHARM)
- Formulation of ATB101 fixed-dose combination product (2017-2018; AUTOTELLICBIO)
- Formulation of ATB102 fixed-dose combination product (2017-2018; AUTOTELLICBIO)
- Physicochemical study of PLLA filler product (2018, PRP SCIENCE)
- Formulation of MRB extended-release oral dosage form (2018; SHINIL PHARM)
- Formulation of APX / RVX solubilized oral dosage form (2018; SHINIL PHARM)
-
Formulation of active coated suture product (2018; me
taBIOMED) - Formulation of TCR solid lipid nanoparticle product (2018; AUTOTELLICBIO)
- Formulation of GMG/DPG fixed-dose combination (2018; LG CHEM)
- 16HBE14o Cell study of AFM/BUD fixed-dose DPI product (2018; KOREA UNITED PHARM)
- Formulation of DNP microneedle patch product (2018; SMALL LAB)
- Lung distribution of AFM/BUD fixed-dose DPI product (2019; KOREA UNITED PHARM)




